TP8
GABAergic neurocognitive defects in RASopathy mouse models
Congenital changes in the RAS-mitogen activated protein kinase (MAPK) signaling pathway can lead to Noonan syndrome, Neurofibromatosis type 1 (NF1), cardiofaciocutaneous syndrome (CFCS) or similar disorders. These RASopathies are characterized by an overlapping pattern of physical abnormalities and variable cognitive impairment. Unfortunately, suitable treatment of cognitive deficits is still hampered by the lack of understanding of the underlying neuronal mechanisms. In this subproject will determine the role of disturbed GABAergic inhibitory function in the development of neurocognitive deficits associated with RASopathy mutations. Using conditional mutant mice will induce selected hyperactivating mutations of the Ras/Raf pathway (PTPN11D61Y, KRASV14I) in GABAergic interneurons and examine their effect on cognitive, emotional and social behavior. GABA-dependent neuronal activity patterns will be studied both in vitro and in vivo, as a proxy of the RASopathy-associated disturbance in information processing. Furthermore, by resolving the mutation-induced intracellular signaling mechanisms in critical subpopulations of these interneurons we aim to identify new therapeutic entry sites.
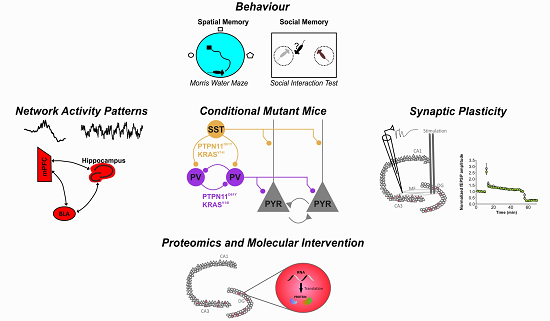
Legend: Mice with interneuron-specific mutation of PTPN11 or KRAS will be in the center of our studies. We will employ behavioral, physiological and molecular investigation of the effects of these RASopathy mutations to determine the mechanisms underlying their cognitive deficits.
Major goals:
- To determine the effects of activating mutations of Ras pathway genes on GABAergic inhibitory interneurons.
- To characterize physiological and behavioral abnormalities induced in transgenic mice by Ras pathway hyperactivation in GABAergic interneurons.
- To explore in transgenic mouse models GABAergic interneurons as entry sites for RASopathy treatment strategies.
TP8 was part of TP2 in previous funding period: project description - previous funding period FP1







