TP7
Exploration of dysregulated lipid metabolism and mitochondrial activity contribution to the RASopathy pathophenotype
In the previous funding period, we found that HRAS G12V CS mice have a reduced life span, display a premature aging-like phenotype, decreased weight gain, a reduced lipid accumulation in the liver and a significant reduction of the white adipose tissue. White adipose tissue reduction is caused by an impaired adipocyte differentiation and increased browning in response to stress, a phenomenon that associates with increased mitochondrial activity and energy expenditure. Furthermore, CS mice liver transcriptomic and proteomic analyses suggest that a constitutive HRAS activation impairs lipid synthesis, enhances catabolism and affects mitochondrial energy metabolism. An elevated energy expenditure under resting conditions was documented also in CS patients and it was speculated to be another pathomechanism underlying the failure to thrive. Thus, we hypothesize that constitutively active HRAS mutations, as well as other RASopathy-associated germline mutations, alter lipid metabolism by both impairing adipogenesis and an increasing lipid catabolism, mitochondrial activity and energy expenditure in both liver and adipose tissue. Ultimately, an impaired energy homeostasis due to an inefficient energy production may well be an additional contributor to the CS-associated failure to thrive.
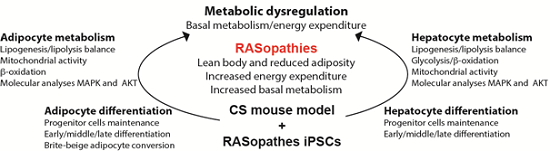
Legend: HRAS germline mutations trigger Costello syndrome (CS), a developmental disorder belonging to RASopathies rare disorders. CS patients are characterized by a reduced life and health span, failure to thrive, features of a mild metabolic wasting syndrome and a premature-like aging phenotype. Our preliminary data identified that energy homeostasis is impaired in a CS mouse model and this can contribute to the general CS pathophenotype.
Major goals:
Making use of the Costello syndrome (CS) HRAS G12V mouse model and of the hepatocytes and adipocytes differentiated in vitro from induced pluripotent stem cells (iPSCs) derived from CS HRAS G12S (provided by SP5), we will pursue the following objectives in detail:
- identifying the onset time of the lipid metabolism dysregulation in the liver and adipose tissue
- delineating the dysfunctional signalling pathways and biological processes implicated in lipid metabolism and mitochondrial activity in
- the CS mouse liver and adipose tissue, and
- in hepatocytes and adipocytes differentiated from RASopathy patient-derived iPSCs
TP7 project description - previous funding period FP1








