TP4 - FP1
From RAS mutations in Noonan syndrome to interaction network profiling and mechanism-based target identification
This subproject focuses on the RAS-MAPK interaction networks in order to explore new molecular and cellular control mechanisms and to identify potential novel RASopathy-relevant targets. Therefore, it was mandatory to establish new and to further develop existing methods, and to optimize culturing of the cell lines of interests, including iPSCs derived from patients with RASopathies and their cardiomyocyte derivatives. A direct approach to identify novel proteins as integral elements of the interaction networks involved in RAS-MAPK signal transduction, such as accessory/scaffold proteins and/or with lipid membrane, is analyzing RAS mutations in codons 5, 50, 58, 71 and 153 identified in individuals with Noonan syndrome. These RAS variants did not exhibit major alterations using so far known `biochemical´ assays under cell-free conditions although they are hyperactive in cells. Another approach is identifying, evaluating and characterizing novel components of the RAS-RAF1 complex as well as novel RAF1 substrates in three independent ways, fractionation combined with 2D blue native PAGE and LC/MS, quantitative SILAC phosphoproteomics and in vitro liposome reconstitution. Functional characterization of newly identified and validated proteins will be crucial to understand the pathogenic mechanisms underlying RASopathy. In addition, we will develop selective cell-based fluorescent probes, which enables us to both monitor in real-time the activity of downstream signaling pathways of RAS and quantitatively measure the activities and specificity of various small molecule inhibitors. These investigations will ultimately disclose further molecular details of dysregulated signaling pathways.
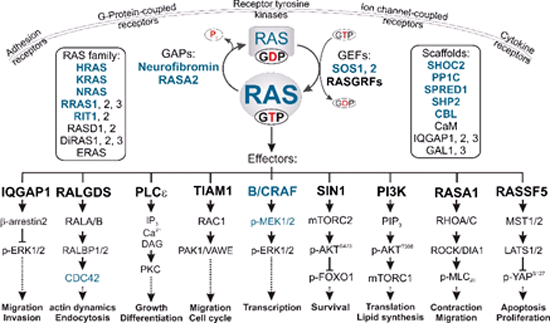
Multiple signal transduction pathways downstream of RAS. The binding of GTP to RAS proteins enables them to undergo high affinity interactions with multiple effectors, which links different extracellular cues to distinct downstream pathways. GEFs and GAPs tightly control two intrinsic reactions, nucleotide exchange and GTP hydrolysis. In a polarized cell, RAS activation and signaling can be accomplished both at different sites of the plasma membrane and in endomembrane compartments. Different members of the RAS family and potential scaffold proteins, potentiating or attenuating signal transduction, are shown in boxes. RASopathy-associated proteins are shown in blue.
Major goals:
- Understanding the RAS interaction networks and identifying and validating new RAS associating proteins as potential new drug targets
- Understanding the impact of the non-conventional RAS variants on the RAS interaction networks
- Investigating of membrane interaction, regulation and signaling of RAS using an in vitro reconstitution model of prenylated RAS proteins on synthetic liposomes
- Establishing fluorescent probes to monitor in real-time RAS-RAF-MEK-ERK and RAS-PI3K-AKT pathways and to decipher critical control mechanisms
Publications:
Altmüller F, Lissewski C, Bertola D, Flex E, Stark Z, Spranger S, Baynam G, Buscarilli de Moraes M, Dyack S, Gillis J, Yntema HG, Pantaleoni F, van Loon RL, MacKay S, Mina K, Schanze I, Tan TY, Walsh M, White SM, Niewisch MR, García-Miñaúr S, Plaza D, Ahmadian MR, Cavé H, Tartaglia M, Zenker M. Genotype and phenotype spectrum of NRAS germline mutations. Eur J Hum Genet. 2017 Jun;25(7):823-831.
PMID: 28594414
Altmüller F, Pothula S, Annamneedi A, Nakhaei-Rad S, Montenegro-Venegas C, Pina-Fernández E, Marini C, Santos M, Schanze D, Montag D, Ahmadian MR, Stork O, Zenker M, Fejtova A. Aberrant neuronal activity-induced signaling and gene expression in a mouse model of RASopathy. PLoS Genet. 2017 Mar 27;13(3):e1006684.
PMID: 28346493
Boppudi S, Bögershausen N, Hove HB, Percin EF, Aslan D, Dvorsky R, Kayhan G, Li Y, Cursiefen C, Tantcheva-Poor I, Toft PB, Bartsch O, Lissewski C, Wieland I, Jakubiczka S, Wollnik B, Ahmadian MR, Heindl LM, Zenker M (2016) Specific mosaic KRAS mutations affecting codon 146 cause oculoectodermal syndrome and encephalocraniocutaneous lipomatosis. Clin Genet. 2016 Oct;90(4):334-42. PMID: 26970110
Nakhaeizadeh H, Amin E, Nakhaei-Rad S, Dvorsky R, Ahmadian MR (2016) The RAS-Effector Interface: Isoform-Specific Differences in the Effector Binding Regions. PLoS One. 2016 Dec 9;11(12):e0167145. PMID: 27936046








